Iodixanol-OptiPrepTM
OptiPrepTM is a ready-made, sterile and endotoxin-tested solution of iodixanol that works across research and clinical workflows, giving you control over your virus purification. Iodixanol can be used for full and empty capsid separation, as well as for AAV particle purification.
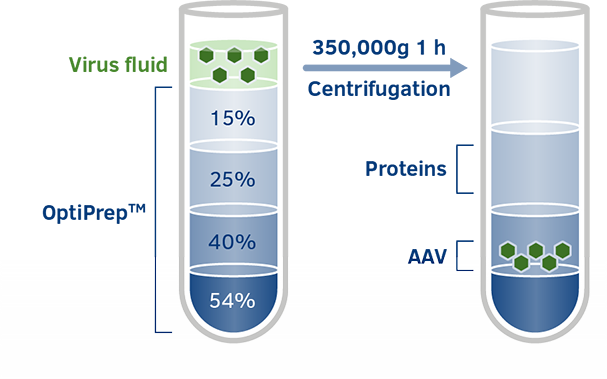
OptiPrepTM for AAV Particle Purification
Iodixanol-OptiPrepTM is an industry standard in AAV gene therapy as a density gradient medium for purifying AAV particles. Density gradient centrifugation has always played an important role in the concentration and purification of virus particles.
Saves time: OptiPrepTM does not cause toxic effects. Therefore, measurements and analyses following purification can be carried out without having to remove OptiPrepTM.
Easy to implement: the same protocol can be used for all serotypes since it is a serotype-independent purification method.
Not destructive: OptiPrepTM causes little or no damage to virus particles due to its isosmotic, non-toxic nature.
Great recovery rate: viruses purified by OptiPrepTM show a high bioactivity, which ensures a high infectivity titer.
Used Industry wide: density gradient centrifugation using OptiPrepTM is an established method to purify AAV particles. In spite of other methods entering the field OptiPrepTM continues to be the go to purification method.

OptiPrepTM for Full and Empty Capsid Separation
Decreasing empty capsids in your AAV preparation minimizes the risk of unwanted immune responses and maximizes transduction efficiency of your AAV gene therapy product making OptiPrepTM an ideal solution for full and empty capsid separation.
Get a competitive advantage: removing empty capsids increases product safety.
Maximize your success rate: improved transduction efficiency allows you to use lower titers.
Reduce patient risk: precise isolation of filled capsids in your gene therapy product to prevent unwanted side effects.
Be ready for clinical trials: focusing on increased safety ensures a smoother transfer.
Manufacture high quality products: by combining with your chromatography manufacturing process.
Clinically tested OptiprepTM: its use in a medical context has been ensured by rigorous testing.
OptiPrepTM Applications
Uses
Beside its use for AAV particle purification and for full and empty capsid separation, OptiPrepTM is commonly used to separate cell types from blood and other tissue e.g. monocytes, lymphocytes, spermatozoa or plant protoplasts.
Due to its chemical features and high quality grade production, it is also suitable for fractionating cell organelles, membranes and ribonucleoproteins, in order to separate viable from non-viable cells or to purify proteins, plasma lipoproteins and plasmid DNA.
Techniques
OptiPrepTM is used for different density gradient designs including discontinuous or continuous gradients due to its high density. It is most commonly used for the preparation of discontinuous gradients.
These kind of gradients can be generated in two specific ways:
-
The most common technique is the overlaying technique. Starting with the densest solution and continuing to layer solutions of successively lower densities on top. To avoid mixing the two adjacent layers of different densities, it is important to place the tip of the pipette or syringe against the wall of the tube and apply the solution in a steady, gentle flow.
-
The second technique is the underlaying technique. Starting with the lowest density solution and underlaying successively denser solutions beneath the lighter solutions. This technique is supposed to be easier than the overlaying technique. However, it is very important to avoid the generation of air bubbles since they disturb the layers and can not very easily be removed.
(Ultra)centrifugation
Whatever technique is chosen to prepare the gradient; the actual purification is performed by (ultra)centrifugation following the application of the sample on top of the gradient. Centrifugation specifications strongly depend on the sample applied. However, always finish with the fractionation of the different components of the sample according to their density.
For many applications such as electrophoresis, infection of cultured cells, in vivo animal experiments, removal of the iodixanol is not a requirement. If for any reason it is required to remove or reduce the iodixanol concentration, a form of ultrafiltration is widely regarded as the most effective method.
OptiPrep FAQs
Yes, it is tested for endotoxins. The endotoxin level is always <1.0 EU/ml usually at ≤ 0.13 EU/ml.
|
|
OptiPrepTM |
CsCl |
|
Osmolality |
Isoosmotic |
High osmolality |
|
Toxicity |
Non toxic to cells |
Highly toxic to cells |
|
Purity of AAV sample1 |
High |
Medium |
|
Time1 |
1 day |
3,5 days |
|
Bioactivity of isolated AAV1 |
Simliar level than CSCl |
Simliar level than Iodixanol, extended exposure to CsCl or variations in sample preparation can significantly reduce bioactivity |
1 Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications
- Ultracentrifugation is not serotype specific and can be used for all serotypes in the same way. It is a universal method.
- Chromatography is more suitable for scale up but needs to be optimized for each serotype. However an iodixanol gradient can be used for full/empty separation with a starting culture of 2000l because the previously performed affinity chromatography reduces volume enormously.
Decreasing empty capsids in your AAV preparation minimizes the risk of unwanted immune responses and maximizes transduction efficiency of your AAV gene therapy product since it allows you to use lower titers. In general removing empty capsids increases product safety which is especially important when preparing for clinical trials.