AAVrh10 Titration ELISA
- ELISA for quantitation of AAVrh10 capsids
- Detection of full and empty AAVrh10 capsids
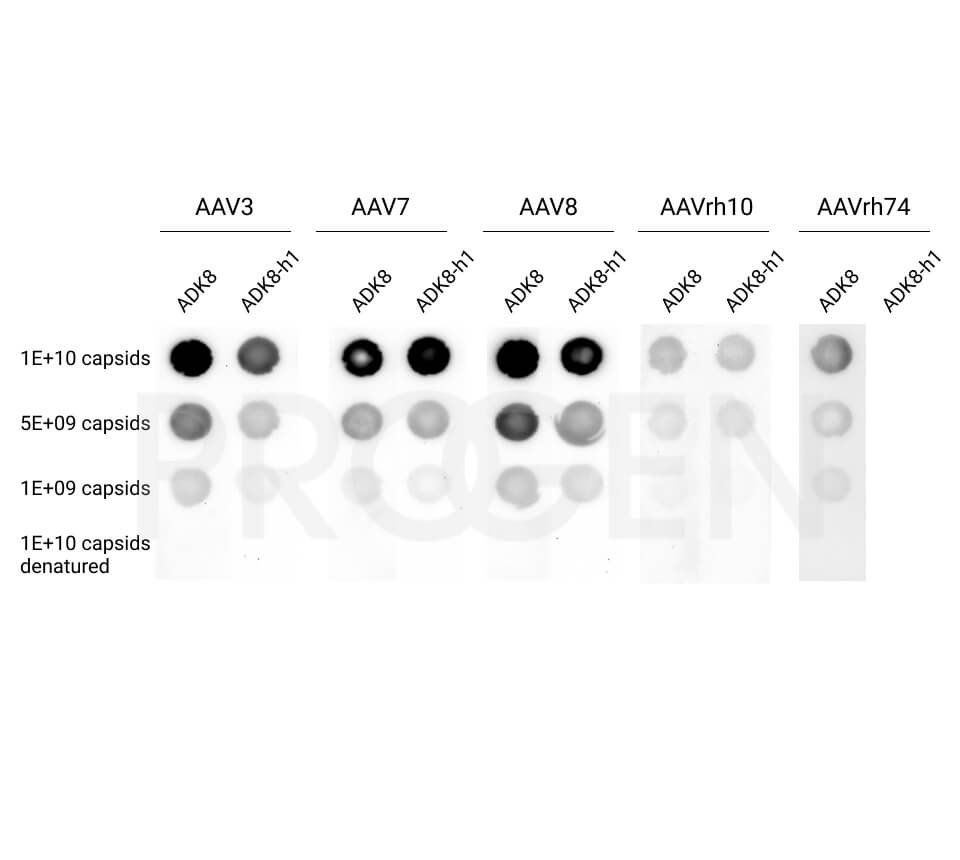
- ADK8 antibody is used as capture and detection antibody
Product description
| Quantity | 96 tests |
|---|---|
| Reactivity | AAVrh10 |
| Storage | 2-8°C |
| Intended use | Research use only |
| Application | ELISA |
| Product description | Unique microtiter plate enzyme immunoassay for the quantitation of virions and assembled empty capsids of AAVrh10. The monoclonal capture-antibody detects a conformational epitope not present on unassembled capsid proteins. |
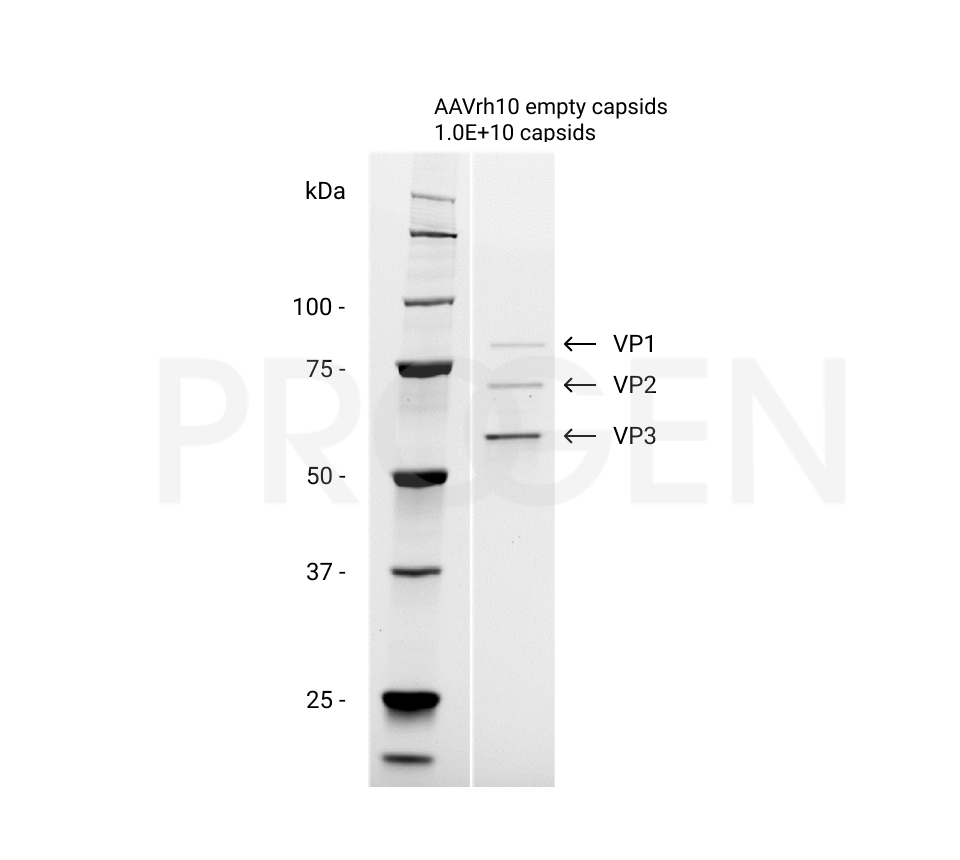
| Details 2 | Empty capsid preparation of AAVrh10 |
| Form supplied | Reagent kit, 12 x 8-well strips |
Background
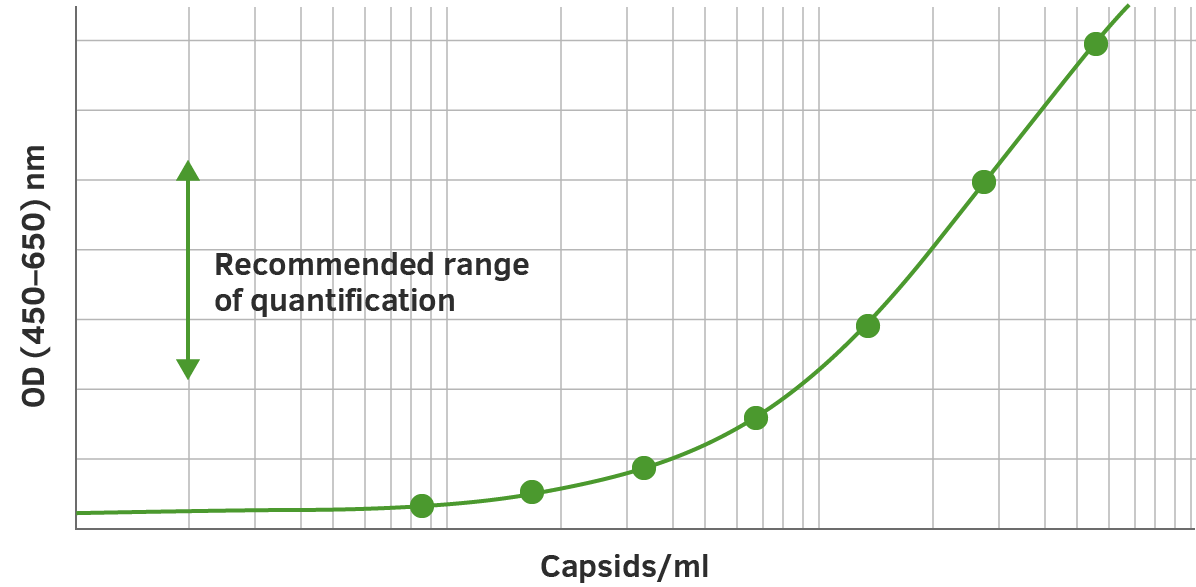
The assay represents a fast, sensitive and reproducible method for titration of intact AAVrh10 wt virions, AAVrh10 recombinant virions or assembled and intact empty AAVrh10 capsids.
The ELISA principle:
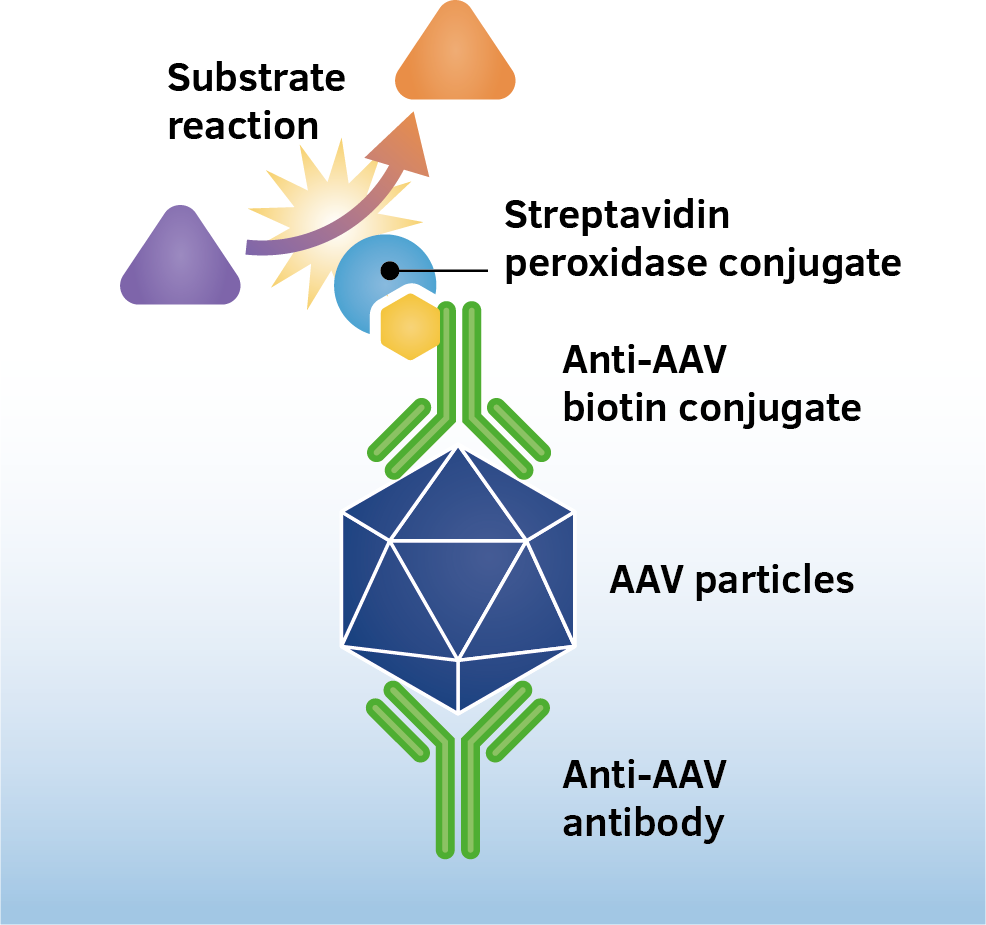
The assay is based on the sandwich ELISA technique where a monoclonal antibody (mab) specific for a conformational epitope on assembled AAV capsids is coated onto the plate and is used to capture AAV particles from the specimen.
The detection of captured AAV particles is a two-step process.
- A biotin-conjugated mab is bound to the captured AAV particles.
- A streptavidin peroxidase conjugate reacts with the biotin molecules. The addition of the substrate results in a color reaction which is proportional to the amount of specifically bound viral particles.
Comparison of AAV Quantification Methods:
Each of the commonly used quantification methods has its pros and cons:
- qPCR is widely used, but suffers from several issues such as sample preparation, primer design, or PCR efficiency that can lead to high inter-laboratory variation of results.
- Digital droplet PCR methods overcome some of the limitations of qPCR. However, variations between labs can still occur due to different sample processing protocols.
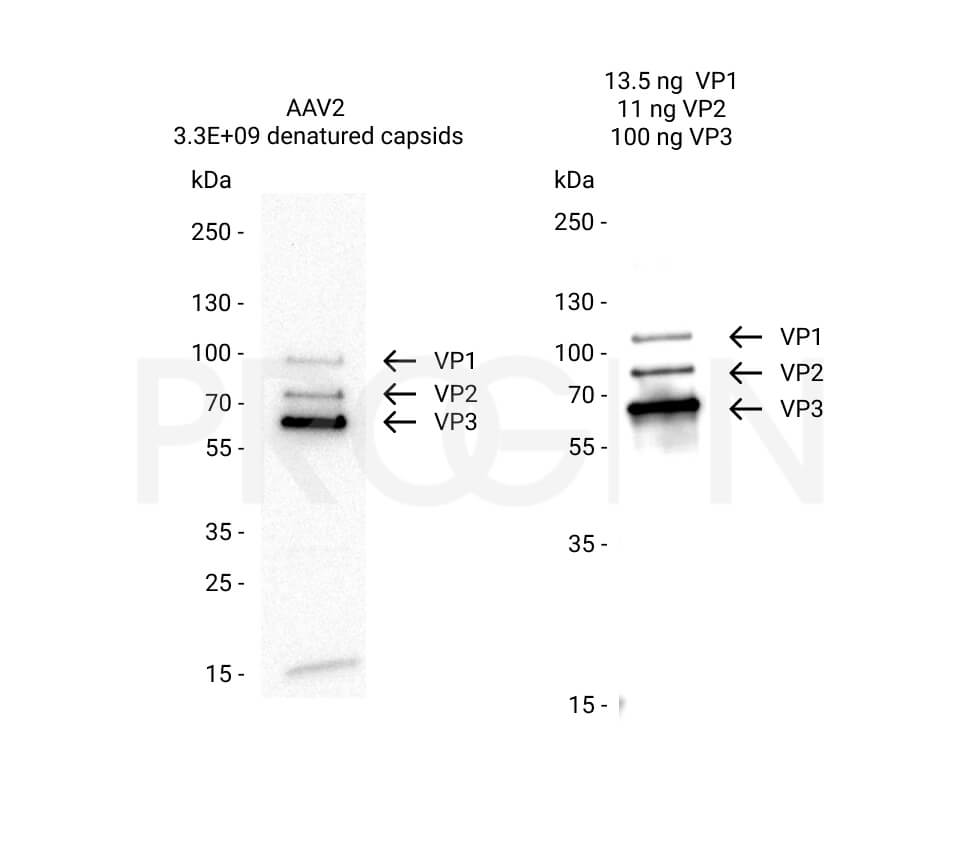
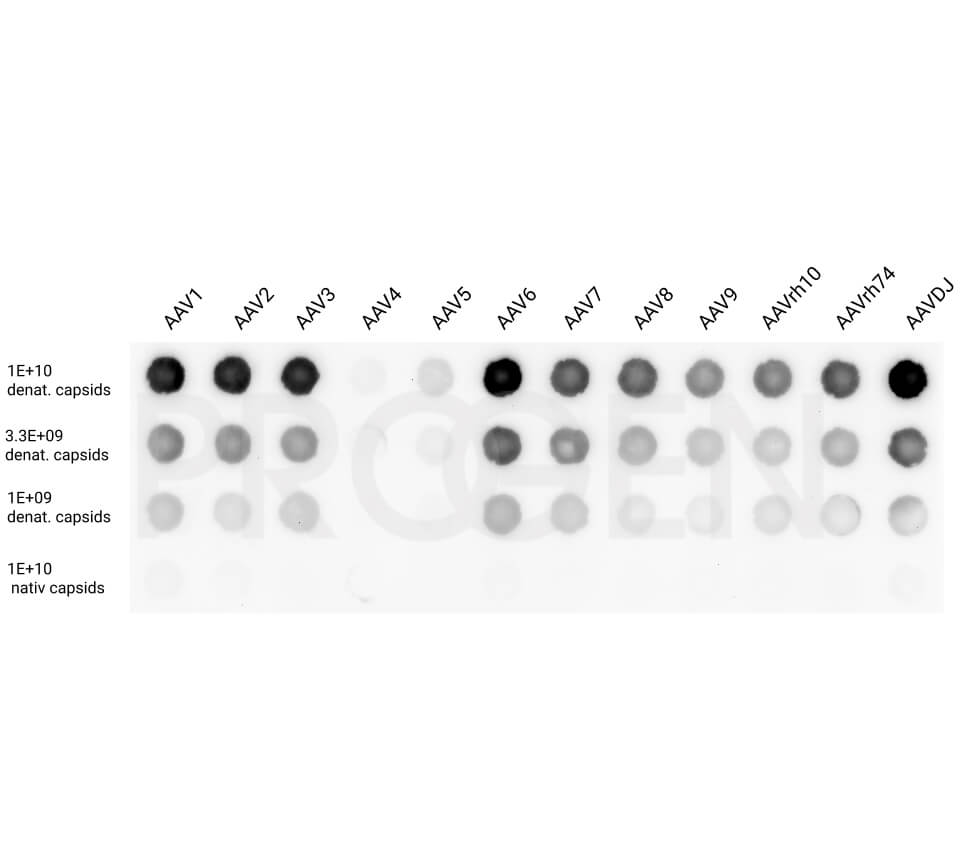
- Dot blot is a simple and quantitative method, if reliable reference material is used. However, it suffers from the limited linearity and dynamic range of western blotting in general.
Given the practical drawbacks of the aforementioned techniques, a conventional sandwich ELISA currently appears to be superior in terms of inter- and intralaboratory variation as well as ease of use. Therefore, it represents the best format for reliable and reproducible quantification of total rAVV capsid titers.
Use of shuffled/mutated AAV:
The recognition of shuffled/mutated AAV vectors depend on the specific capsid region which is affected by the shuffling/mutation. The capture antibodies used for PROGEN’s AAV ELISAs bind specific and, in some cases, well defined conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes. A first indication that the ELISA might recognize your shuffled/mutated AAV vector is the presence of the antibody-binding epitope. However, changes in the protein sequences of the capsid proteins might also influence conformation of the proteins, hence the conformation of the epitopes presented on the AAV capsid. This might influence binding affinity of the antibody and affect determination of the titer based on the (non-shuffled) Kit Control provided with the AAV ELISA kit. Since these properties strongly depend on the specific shuffling/mutation performed, PROGEN cannot guarantee successful and precise quantification of your shuffled/mutated AAV vector. Even if the antibody binding epitopes are still present on your shuffled/mutated AAV capsid, your assay needs to be tested and optimized for your specific AAV vector.
PROGEN highly recommends the production and calibration of a suitable (shuffled/mutated) Kit Control, to ensure reliable titer determination of your individually shuffled/mutated AAV vector with PROGEN ELISA kits.
Limited Use Label License: Research Use Only
Product is exclusively owned by PROGEN Biotechnik GmbH. The use of these products for the development, manufacturing and sale of secondary products/derivatives which are based on the purchased products and/or which include the purchased product require a royalty based sub-license agreement.
Downloads
Q & A's
Customer Reviews
Login
FAQs
Performance data is available here.
The recognition of shuffled AAV vectors depends on the specific capsid region which is affected by the shuffling. The antibodies used for PROGEN’s AAV ELISAs bind to specific conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes.
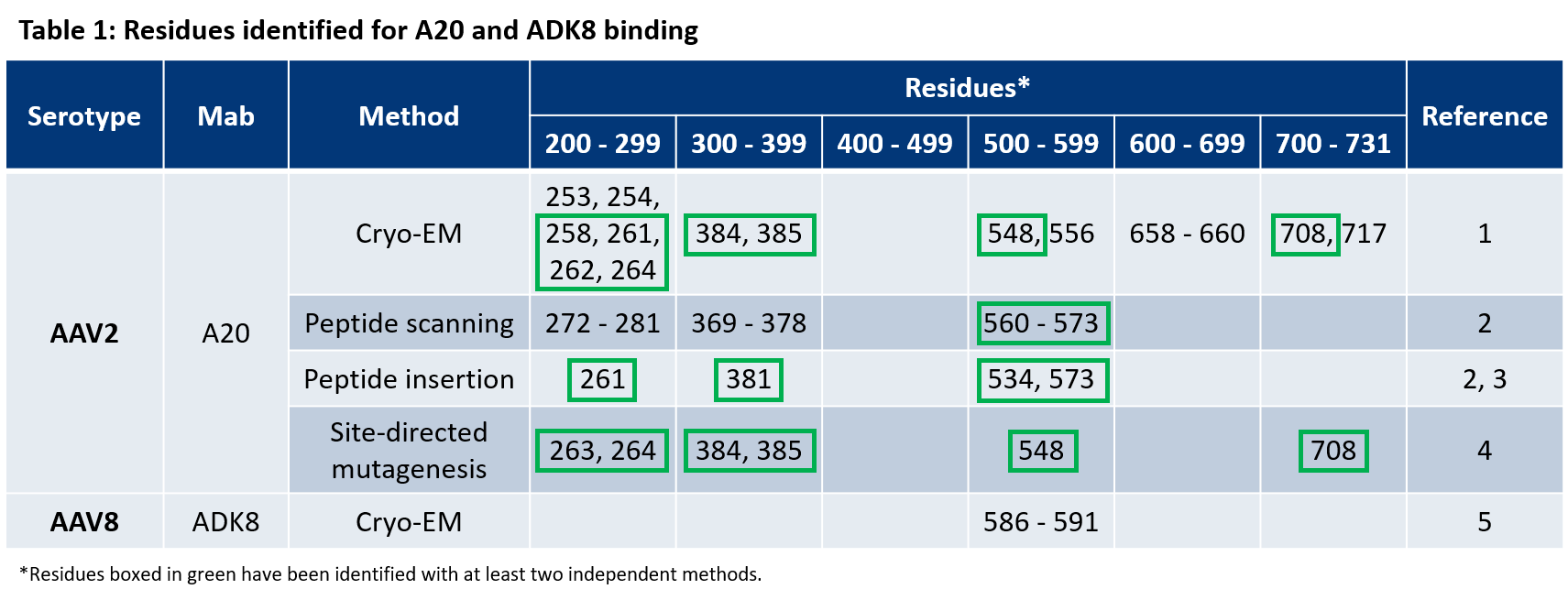
- McCraw et al. Structureof adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology (2012) 431:40-9.
- Wobus, C. E. et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74, 9281–93 (2000).
- Huttner et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther (2003) 10:2139-47.
- Lochrie et al. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol (2006) 80:821-34.
Due to the high affinity and specificity of the AAV capture antibodies used for the PROGEN AAV ELISA kits, the detection of AAV particles from cell extracts is possible. However, detection is only possible within the reading range of the ELISA kits and reliable quantification depends on adequate titration of the corresponding samples. Furthermore, the detection of AAV capsids from cell extract can be influenced by several conditions, e.g. the composition of your lysis buffer. For example, high salt concentrations in your buffer might inhibit adequate capsid detection. We recommend to dilute the sample at least 1:10 in ASSB 1x to get a reliable result. For more information, see the following publication:
Yes, PROGEN offers serotype-specific AAV ELISA Controls for the standardization of your AAV titer determination.
The serotype-specific positive controls are fully characterized empty AAV capsids standardized with PROGEN´s internal gold standards* (AAV1, AAV3, AAV5, AAV6, AAV9, AAVrh10 and AAVrh74) or the ATCC international gold standard material (AAV2 & AAV8).
*For more information on PROGEN´s internal gold standard, take a look at our posters "Developing Reliable AAV Standards for ELISA" and "The New AAV3 Titration ELISA" describing the establishment and characterization of AAV5 and AAV3 internal gold standards.
Our Dip'n'Check AAV lateral flow assays are perfectly suitable to estimate the approximate titer quick and easy just before performing the PROGEN ELISA. The result within 20min will guide you to the optimal dilution for your sample to hit the dynamic range. More information about our Dip’n’Check can be found on our Poster: Potential Applications for AAV Lateral Flow Tests
Yes, for AAV particle purification we recommend OptiPrep produced by Serumwerk Bernburg AG. You can find more information on our OptiPrep site.
Bearing this in mind, once you have applied the standard curve, forty duplicate measurements can be applied.
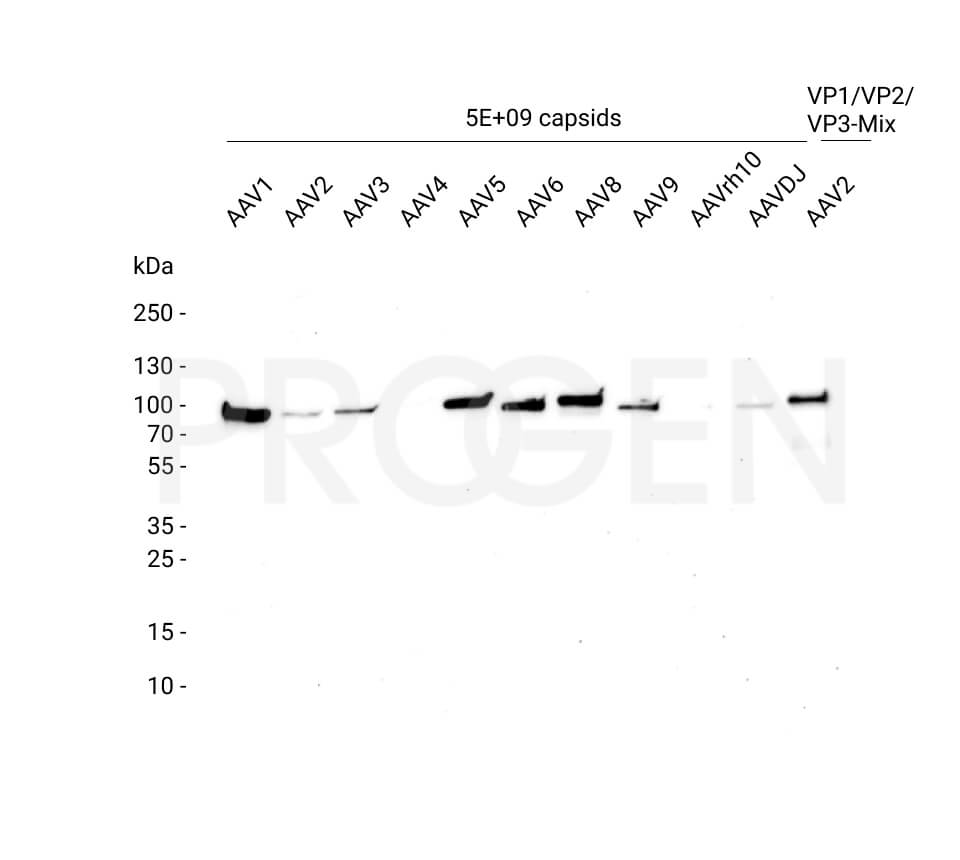
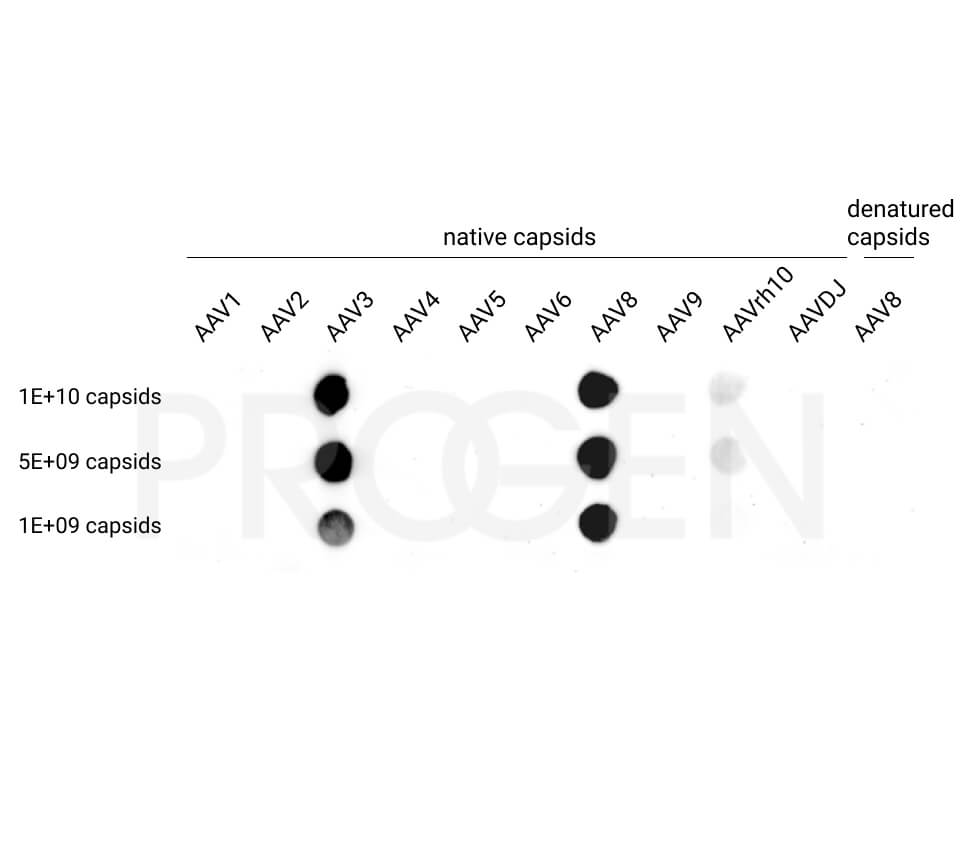
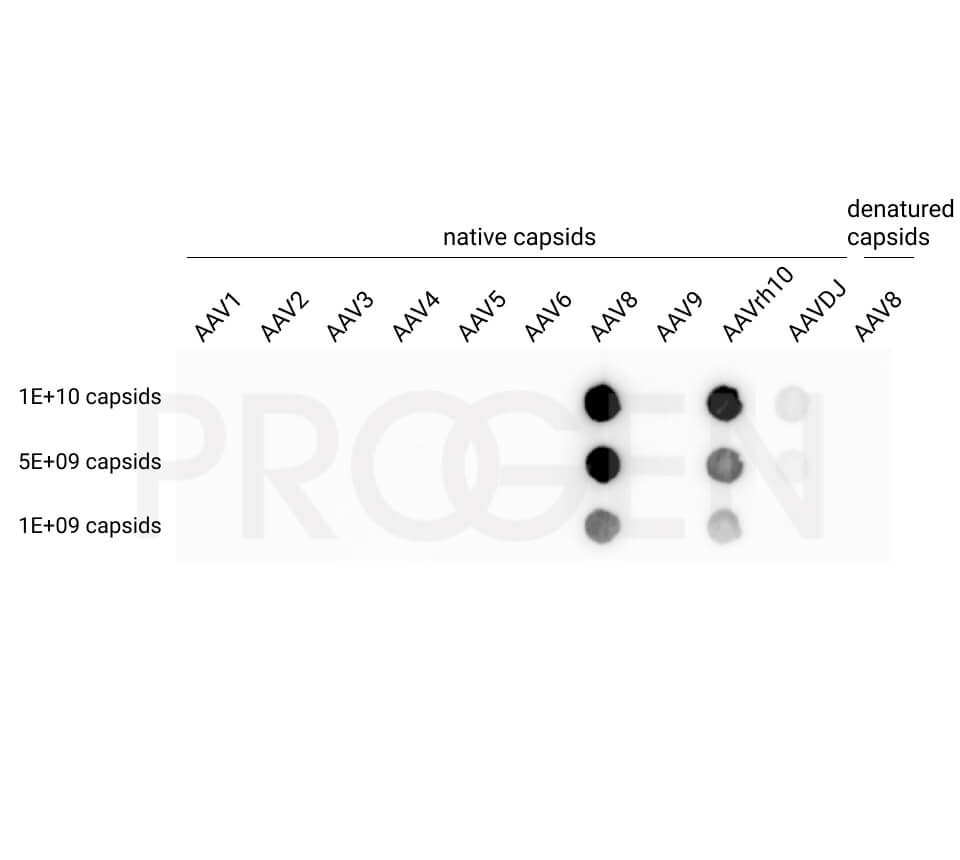
PROGEN offers AAV Titration ELISA kits for the quantification of AAV serotypes 1, 2, 3, 5, 6, 8, 9, and rh10 and AAV Xpress ELISA kits for AAV serotypes 2, 5, 6, 8, and 9. AAVrh74 capsids can be quantified with a combination of AAVrh10 ELISA and AAVrh74 kit control as standard, since the antibody used in the AAVrh10 ELISA also recognizes AAVrh74.
Yes, some of the antibodies used in our ELISA kits show cross-reactivity with other serotypes.
For more information on cross-reactivity of our antibodies, please visit our AAV particle antibody webpage.
For example, the AAV8 ELISA kits cross-react with AAVrh10. However, the AAV8 ELISA kit is not suitable for quantification of the total AAV capsid content of AAVrh10 preparations e.g. due to its serotyp-specific kit control. For quantification of AAVrh10 PROGEN offers an ELISA optimized for AAVrh10 containing AAVrh10 kit control.
With a suitable kit control an ELISA kit can be validated for a specific serotyp. For example, PROGEN offers the AAVrh74 Kit control, which can be used in combination with our AAVrh10 ELISA kit to quantify AAVrh74 capsids. This specific applications needs to be qualified by the user.
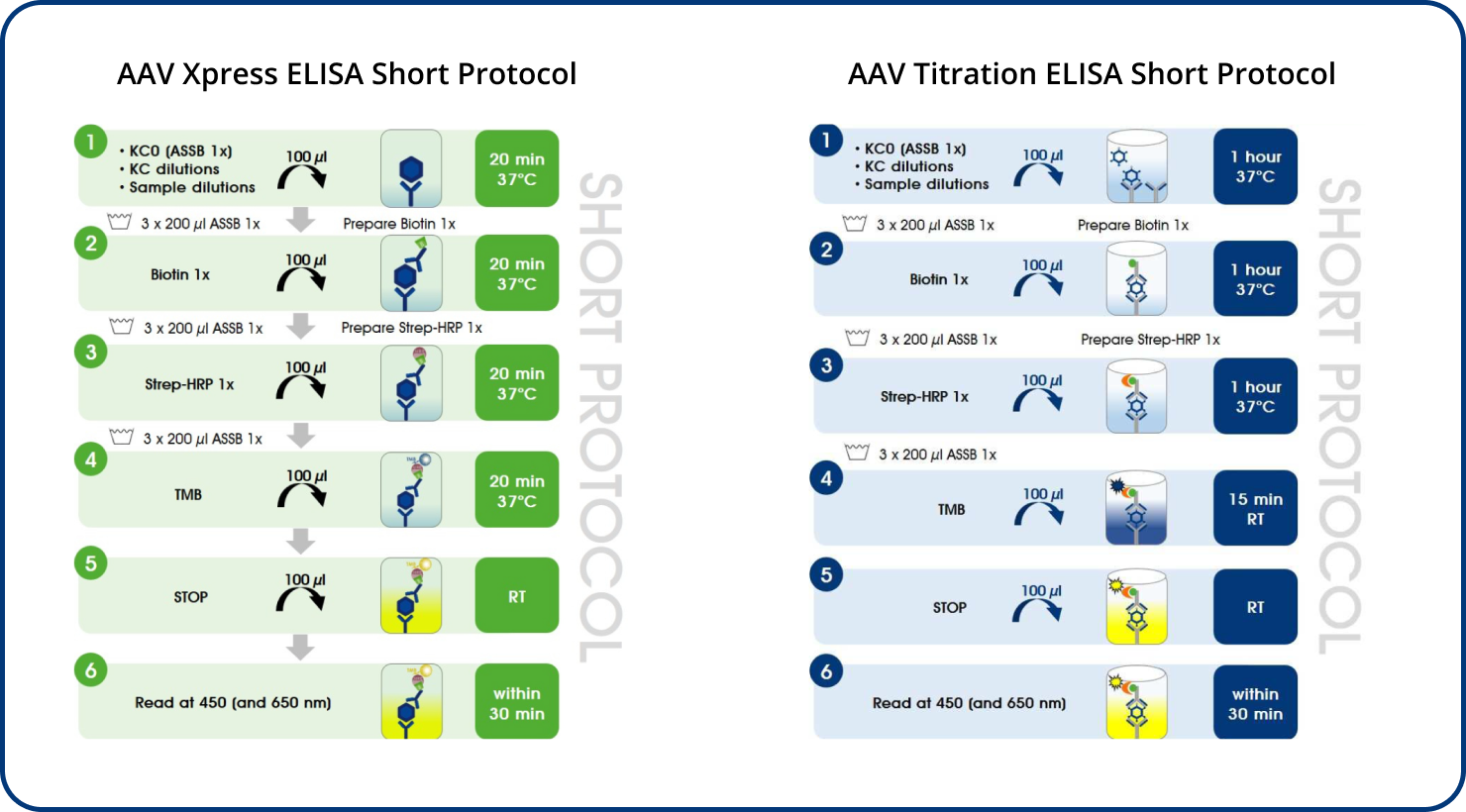
AAV Xpress ELISAs were developed based on the corresponding AAV Titration ELISAs.