"In the community of lipid droplet biology research, your antibodies are highly appreciated and many papers in this field rely on PROGEN antibodies."
Prof. Dr. Matthijs Hesselink, Maastricht University Medical Center, The Netherlands
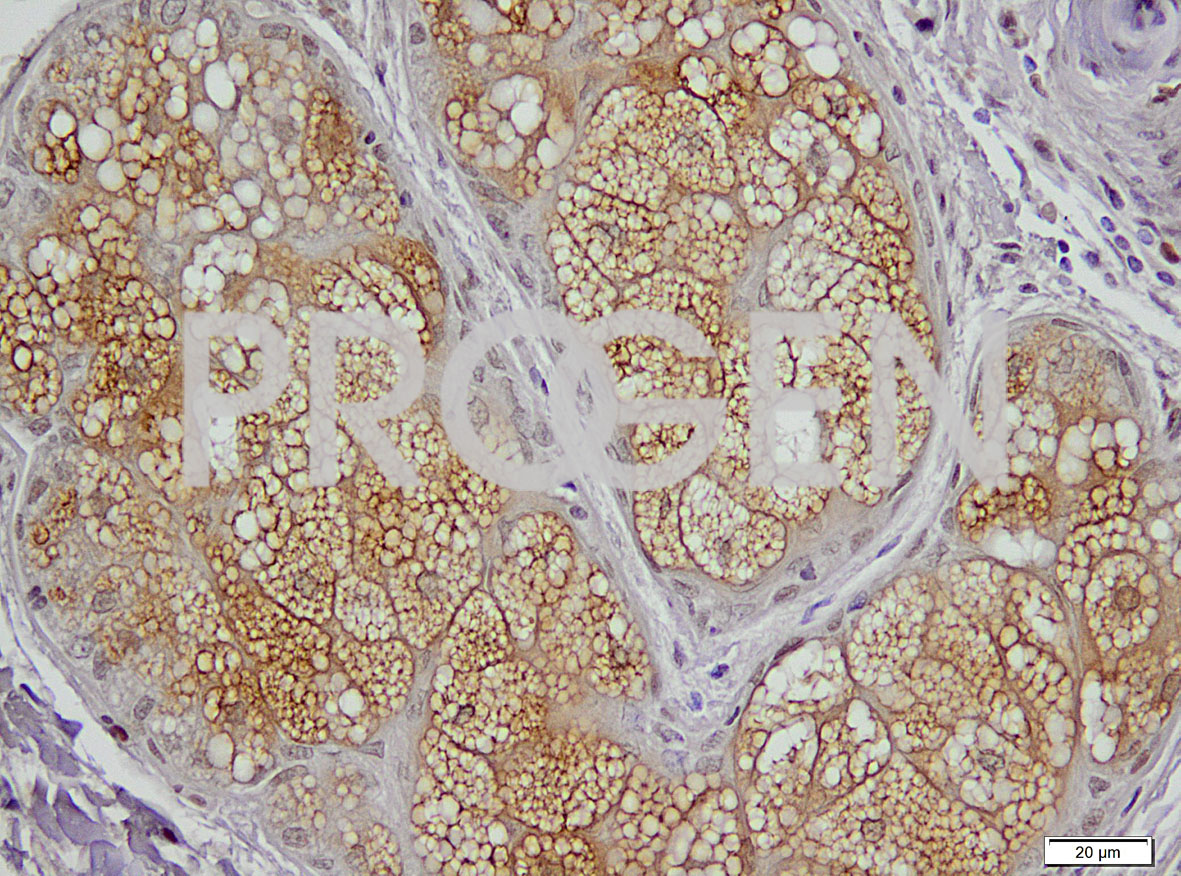
Perilipin-2 immunohistochemistry of human skin; © PROGEN in cooperation with Uniklinik Heidelberg, Dr. Jochen Heß
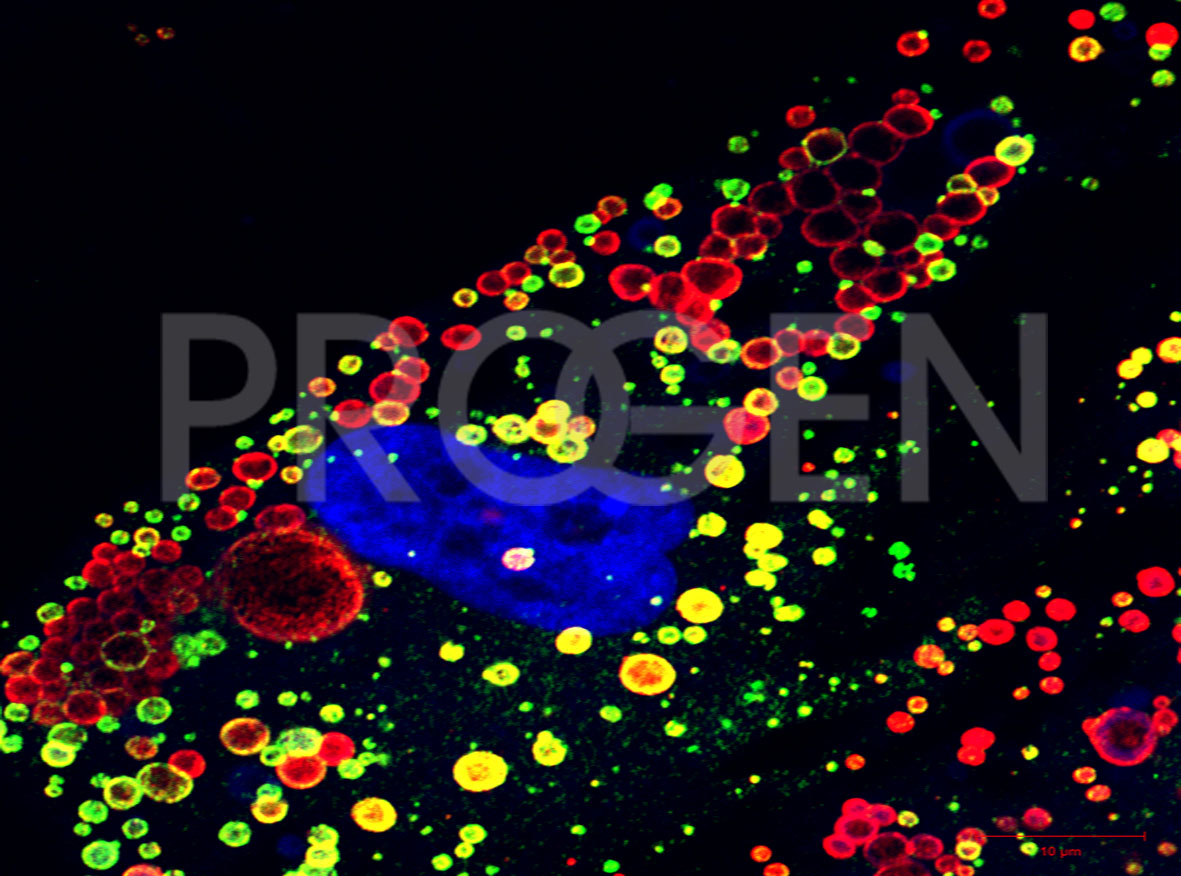
Immunofluorescent double-staining of perilipin-1 (red) and perilipin-2 (green) in human preadipose cells, DAPI-labelled nucleus (purple); © W. Franke, DKFZ Heidelberg


