AAV ELISA FAQs
Performance data is available here.
Yes, some of the antibodies used in our ELISA kits show cross-reactivity with other serotypes.
For more information on cross-reactivity of our antibodies, please visit our AAV particle antibody webpage.
For example, the AAV8 ELISA kits cross-react with AAVrh10. However, the AAV8 ELISA kit is not suitable for quantification of the total AAV capsid content of AAVrh10 preparations e.g. due to its serotyp-specific kit control. For quantification of AAVrh10 PROGEN offers an ELISA optimized for AAVrh10 containing AAVrh10 kit control.
With a suitable kit control an ELISA kit can be validated for a specific serotyp. For example, PROGEN offers the AAVrh74 Kit control, which can be used in combination with our AAVrh10 ELISA kit to quantify AAVrh74 capsids. This specific applications needs to be qualified by the user.
The recognition of shuffled AAV vectors depends on the specific capsid region which is affected by the shuffling. The antibodies used for PROGEN’s AAV ELISAs bind to specific conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes.
- McCraw et al. Structureof adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology (2012) 431:40-9.
- Wobus, C. E. et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74, 9281–93 (2000).
- Huttner et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther (2003) 10:2139-47.
- Lochrie et al. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol (2006) 80:821-34.
Yes, PROGEN offers serotype-specific AAV ELISA Controls for the standardization of your AAV titer determination.
The serotype-specific positive controls are fully characterized empty AAV capsids standardized with PROGEN´s internal gold standards* (AAV1, AAV3, AAV5, AAV6, AAV9, AAVrh10 and AAVrh74) or the ATCC international gold standard material (AAV2 & AAV8).
*For more information on PROGEN´s internal gold standard, take a look at our posters "Developing Reliable AAV Standards for ELISA" and "The New AAV3 Titration ELISA" describing the establishment and characterization of AAV5 and AAV3 internal gold standards.
Yes, for AAV particle purification we recommend OptiPrep produced by Serumwerk Bernburg AG. You can find more information on our OptiPrep site.
Our Dip'n'Check AAV lateral flow assays are perfectly suitable to estimate the approximate titer quick and easy just before performing the PROGEN ELISA. The result within 20min will guide you to the optimal dilution for your sample to hit the dynamic range. More information about our Dip’n’Check can be found on our Poster: Potential Applications for AAV Lateral Flow Tests
Bearing this in mind, once you have applied the standard curve, forty duplicate measurements can be applied.
Due to the high affinity and specificity of the AAV capture antibodies used for the PROGEN AAV ELISA kits, the detection of AAV particles from cell extracts is possible. However, detection is only possible within the reading range of the ELISA kits and reliable quantification depends on adequate titration of the corresponding samples. Furthermore, the detection of AAV capsids from cell extract can be influenced by several conditions, e.g. the composition of your lysis buffer. For example, high salt concentrations in your buffer might inhibit adequate capsid detection. We recommend to dilute the sample at least 1:10 in ASSB 1x to get a reliable result. For more information, see the following publication:
AAV Xpress ELISAs were developed based on the corresponding AAV Titration ELISAs.
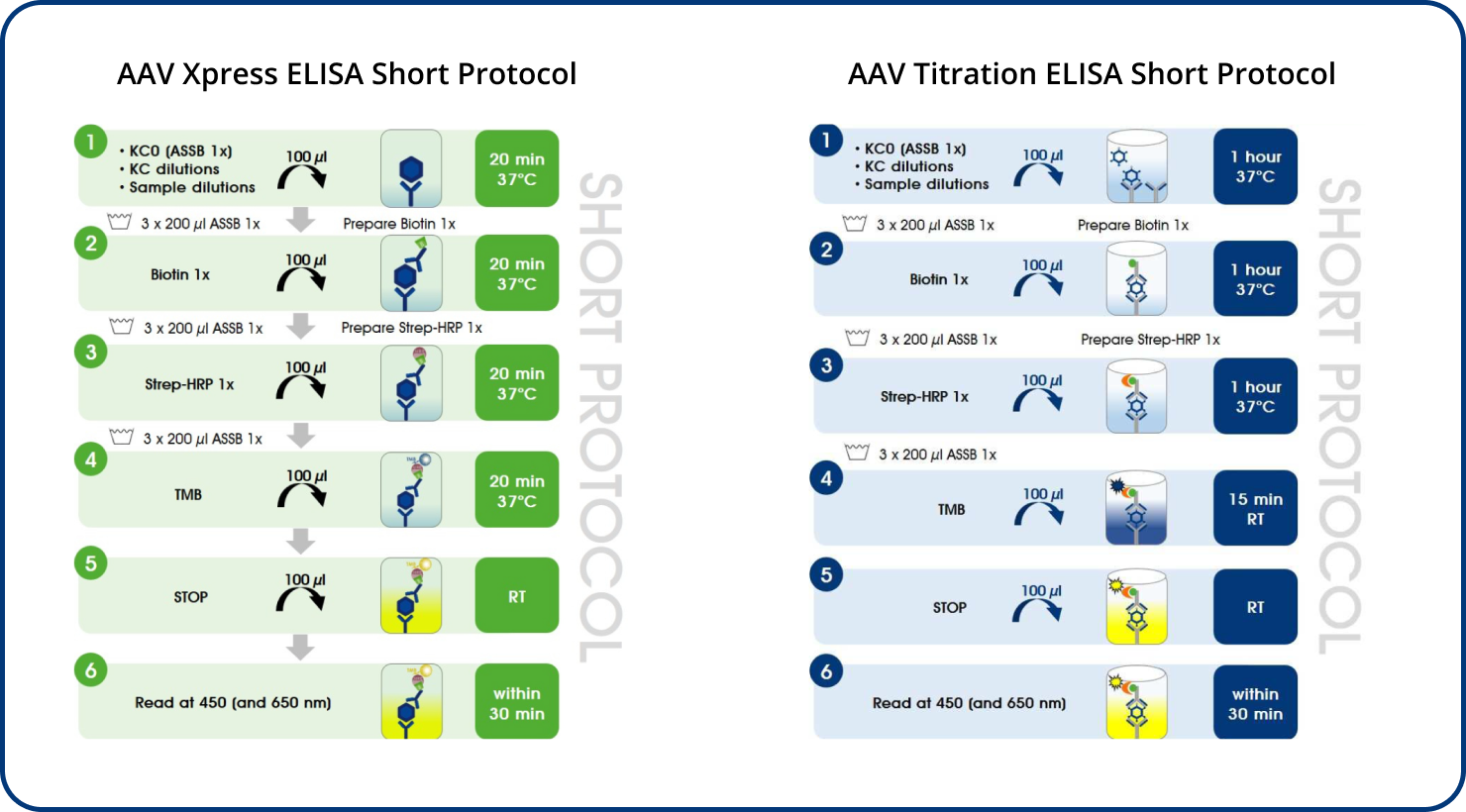
PROGEN offers AAV Titration ELISA kits for the quantification of AAV serotypes 1, 2, 3, 5, 6, 8, 9, and rh10 and AAV Xpress ELISA kits for AAV serotypes 2, 5, 6, 8, and 9. AAVrh74 capsids can be quantified with a combination of AAVrh10 ELISA and AAVrh74 kit control as standard, since the antibody used in the AAVrh10 ELISA also recognizes AAVrh74.
AAV Antibodies FAQs
Yes, our AAV particle antibodies detect conformational epitopes only present on assembled capsids.
You can find all AAV particle antibodies here.
In contrast, AAV capsid protein antibodies recognize linear epitopes present on denatured VP proteins.
You can find all AAV capsid protein antibodies here.
Yes, PROGEN offers a selection of AAV antibodies in 10 µg sample sizes. You can find all available AAV antibodies here: AAV Antibodies
Anti-Adeno-Associated Virus Capsid Proteins Antibodies
Anti-Adeno-Associated Virus Particle Antibodies
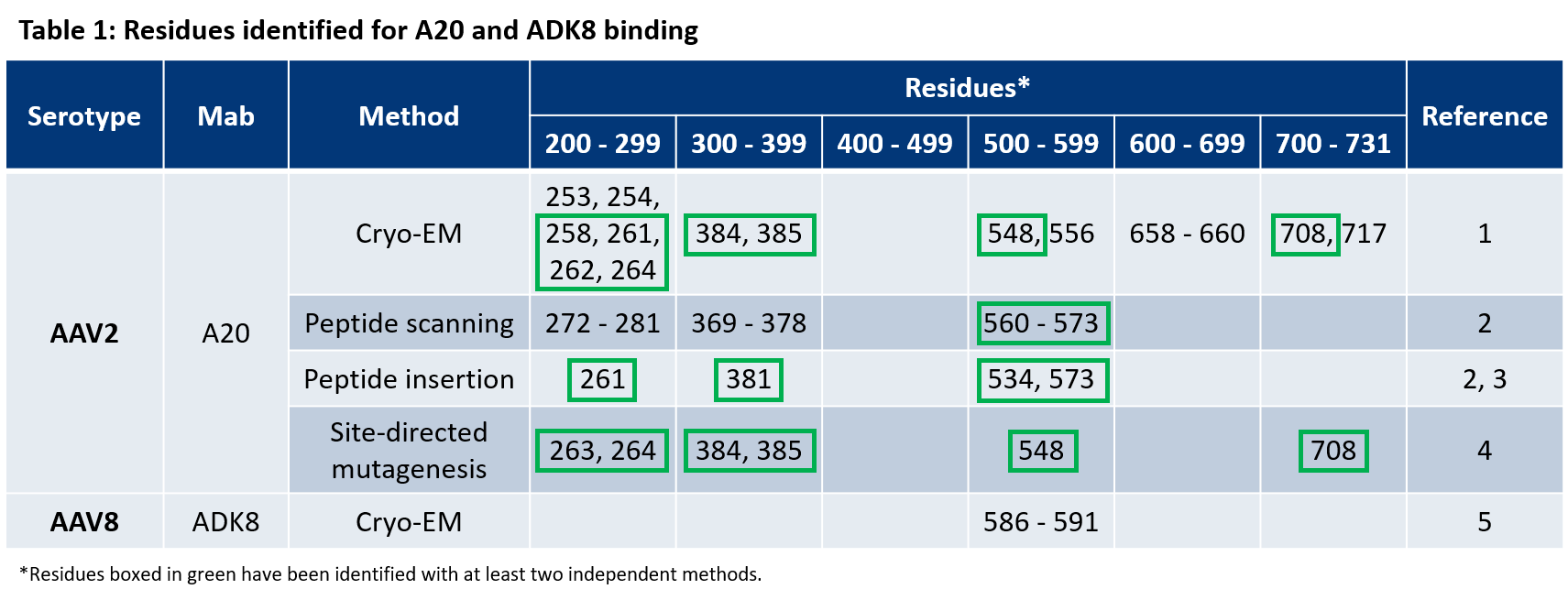
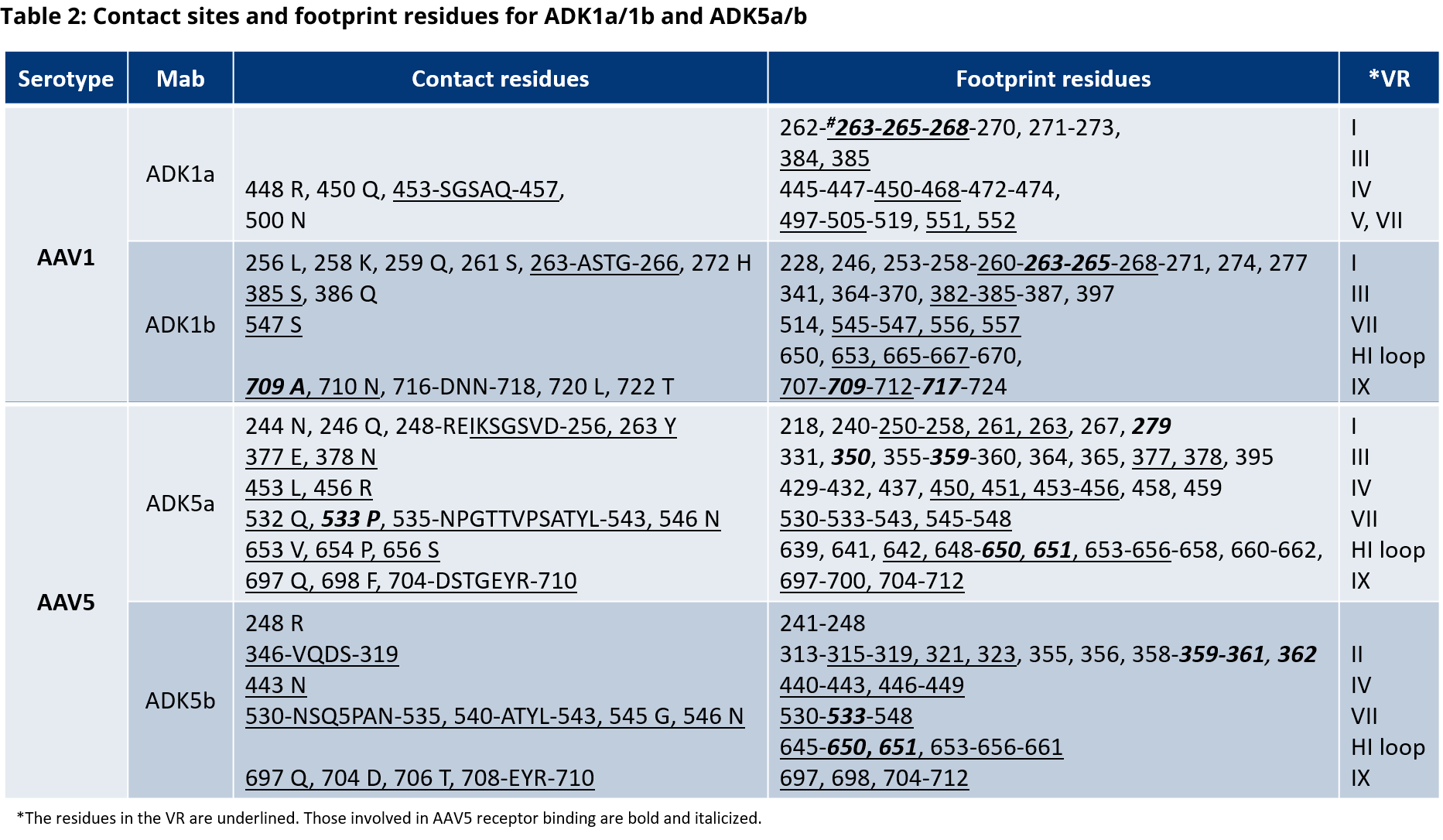
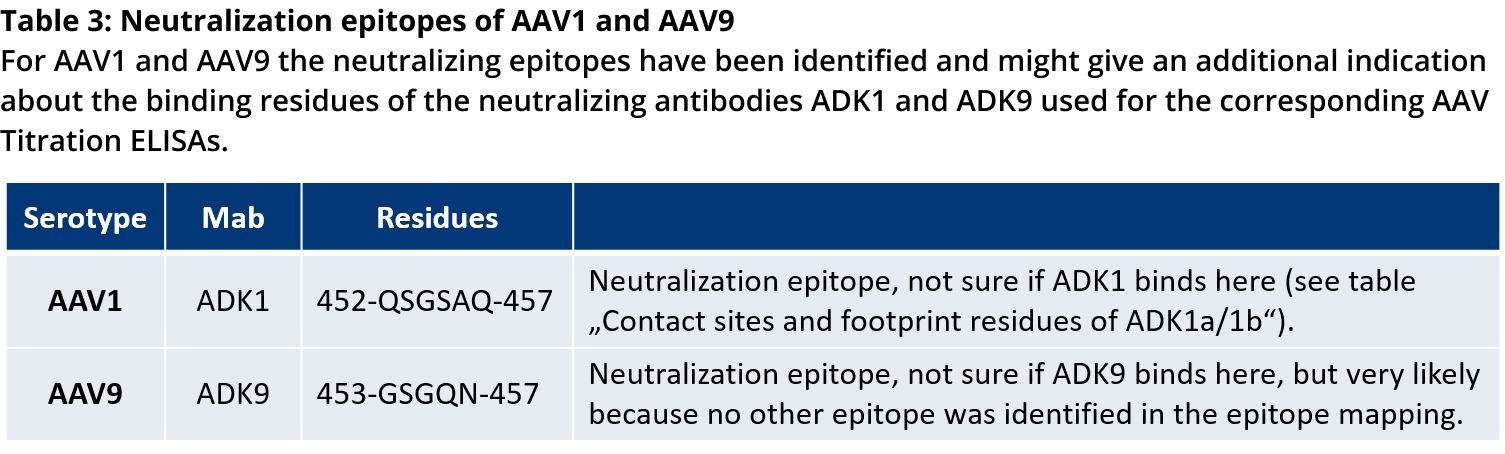
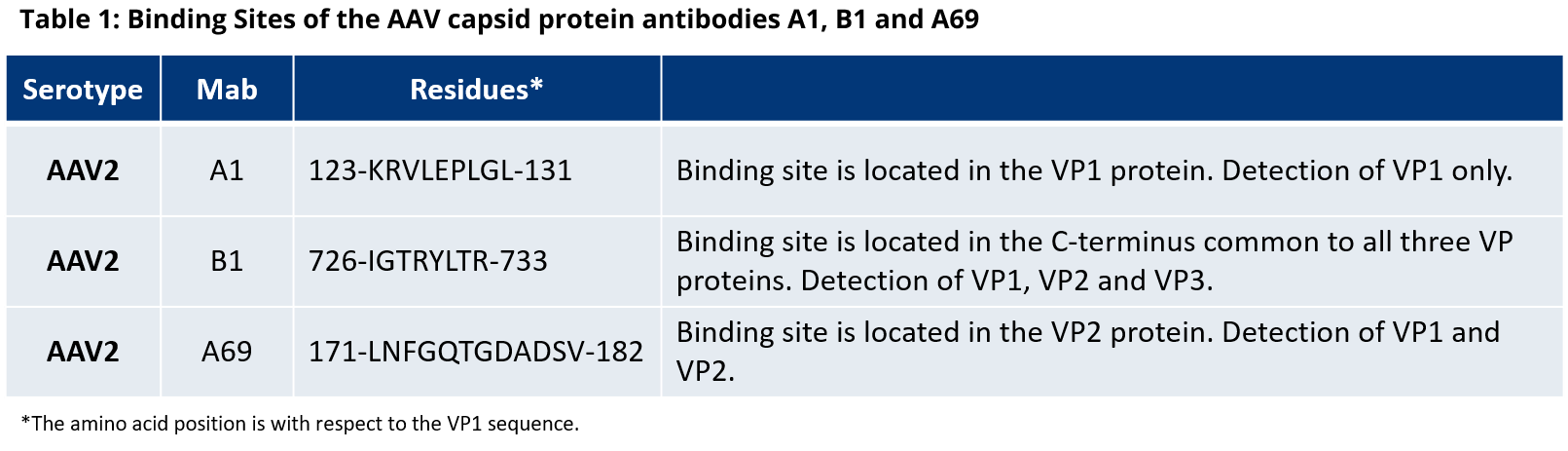
For more information on the antibody binding epitopes on assembled AAV1, 2, 5, 8 and 9 capsids, see the tables below summarizing the published data from the following publications on the specific antibody binding sites: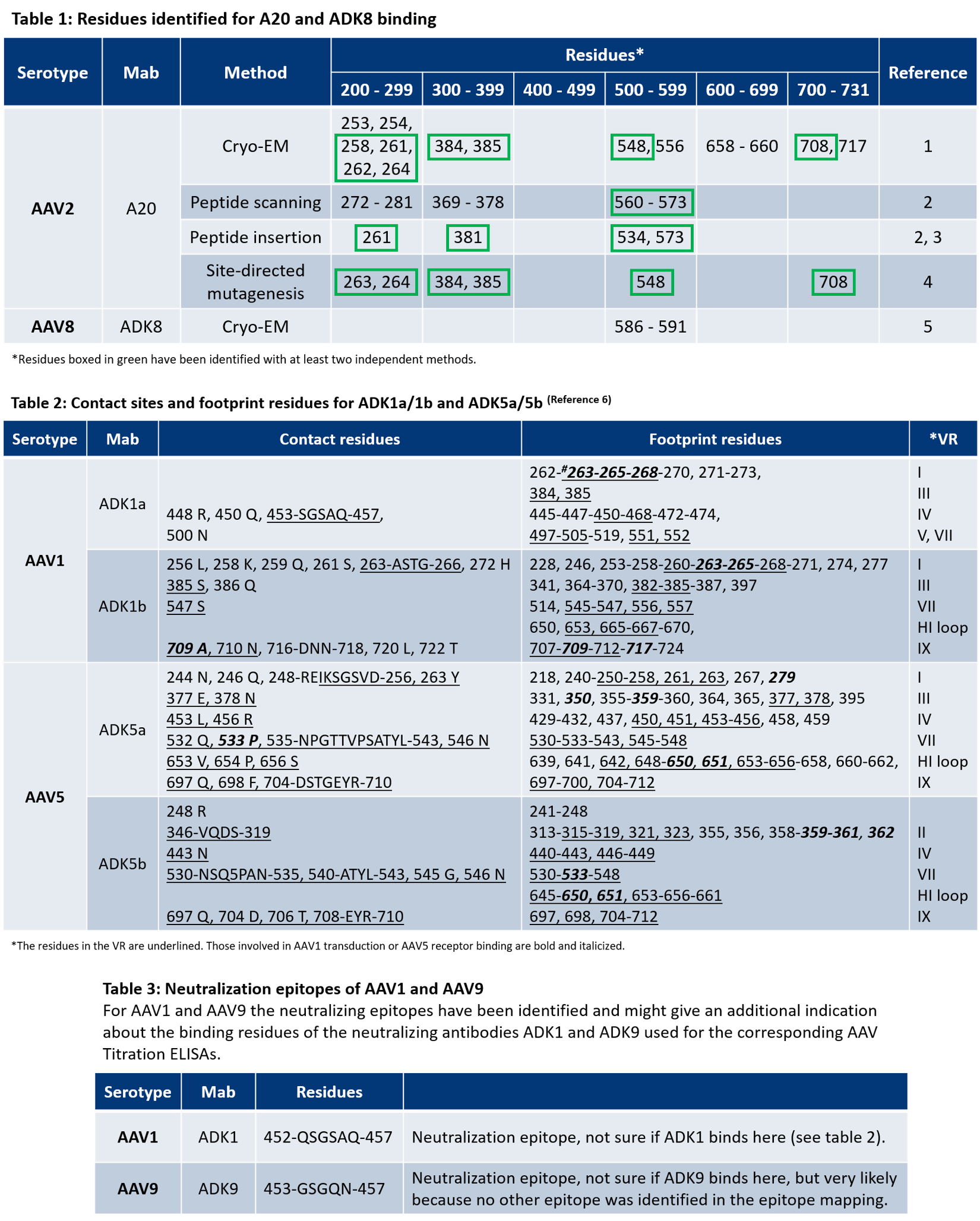
-
AAV 1
- Tseng, Y.-S. et al. Adeno-Associated Virus Serotype 1 (AAV1)-and AAV5-Antibody Complex Structures Reveal Evolutionary Commonalities in Parvovirus Antigenic Reactivity. J. Virol. 89, 1794–1808 (2015).
- McCraw et al. Structureof adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology (2012) 431:40-9.
- Wobus, C. E. et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74, 9281–93 (2000).
- Huttner et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther (2003) 10:2139-47.
- Lochrie et al. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol (2006) 80:821-34.
- Tseng, Y.-S. et al. Adeno-Associated Virus Serotype 1 (AAV1)-and AAV5-Antibody Complex Structures Reveal Evolutionary Commonalities in Parvovirus Antigenic Reactivity. J. Virol. 89, 1794–1808 (2015).
- Gurda, B. L. et al. Mapping a Neutralizing Epitope onto the Capsid of Adeno-Associated Virus Serotype 8. J. Virol. 86, 7739–7751 (2012).
EM structures of assembled AAV1, 2, 4, 5, 6, and 8 capsids with Fab-fragments are available in the following publications:
- Emmanuel, S. N. et al. Parvovirus Capsid-Antibody Complex Structures Reveal Conservation of Antigenic Epitopes Across the Family. Viral Immunolgoy. 34(1) (2021).
- McCraw, S. M. et al. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology. 432(1-2):40-9 (2012).
- Tseng, Y. et al. Adeno-Associated Virus Serotype 1 (AAV1)- and AAV5-Antibody Complex Structures Reveal Evolutionary Commonalities in Parvovirus Antigenic Reactivity. J Virol. 89(3):1794-1808 (2015).
- Jose, A. et al. High-Resolution Structural Characterization of a New Adeno-associated Virus Serotype 5 Antibody Epitope toward Engineering Antibody-Resistant Recombinant Gene Delivery Vectors. J Virol. 93(1):e01394-18 (2019).
- Silveria, M. A. et al. The Structure of an AAV5-AAVR Complex at 2.5 Å Resolution: Implications for Cellular Entry and Immune Neutralization of AAV Gene Therapy Vectors. Viruses. 12(11):1326 (2020).
