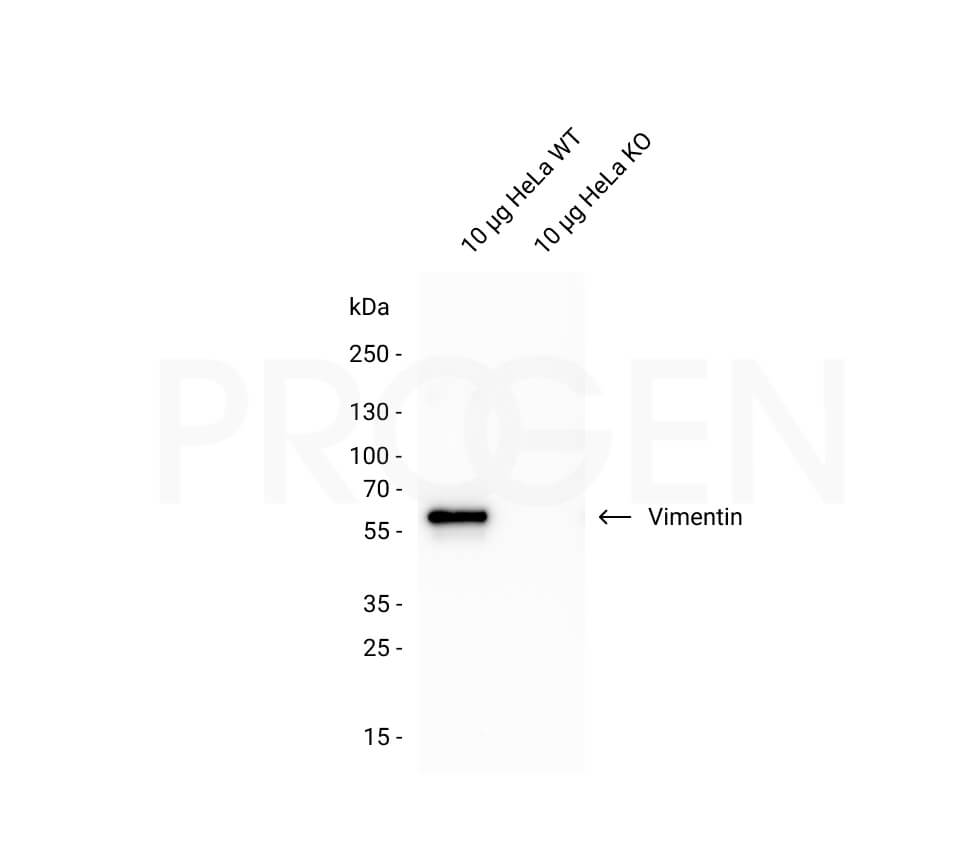
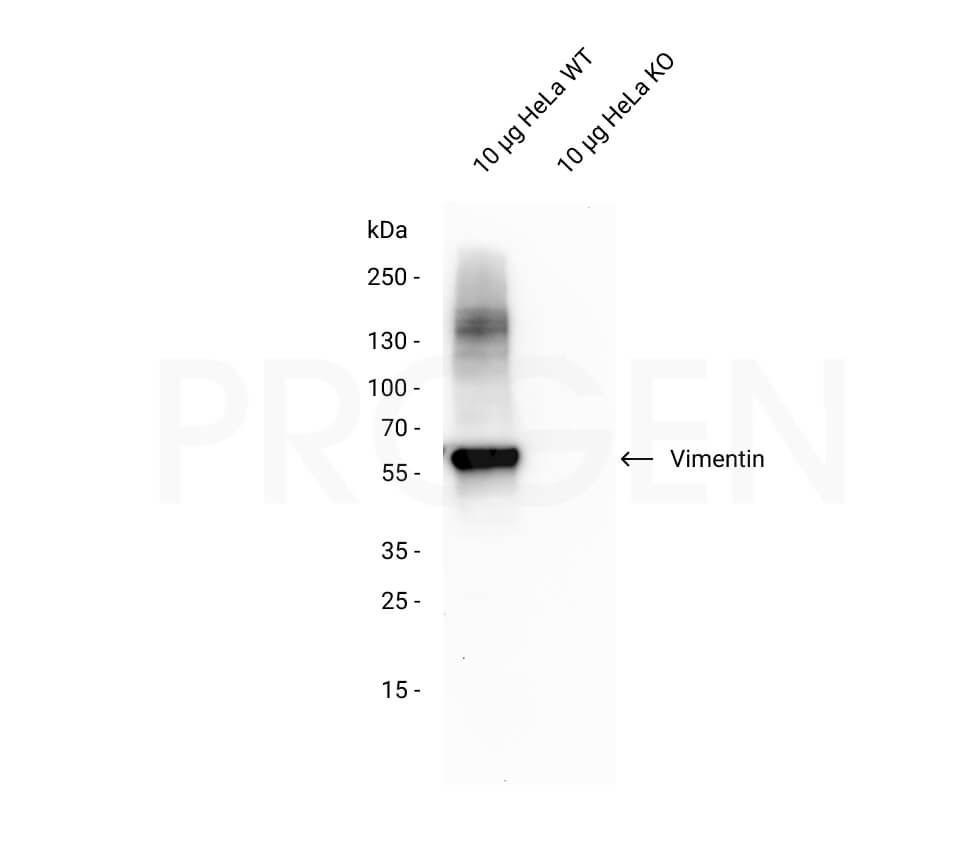
Vimentin, human recombinant, 100 µg
Key Features
- Recombinant human vimentin
- Protein standard
Product description
| Quantity | 100 µg |
|---|---|
| Storage | Lyophilized at 2-8°C; reconstituted at -20°C (avoid freeze/thaw cycles) |
| Intended use | Research use only |
| Source | Human recombinant, produced in E. coli |
| Molecular weight | 57 kDa |
| Isoeletric point | pI 5.3 |
| Purity | > 95% (determined by SDS gelelectrophoresis) |
| Reconstitution | Reconstitute with 70 µl distilled water (final volume 100 µl). Final solution: 30 mM Tris/HCI pH 8, 9.5 M urea, 2 mM DTT, 2 mM EDTA, 10 mM methylammonium chloride; Protein concentration: 1 mg/ml |
| Application | Protein standard in 1D and 2D SDS gelelectrophoresis, immunoassays and immunization |
Background
Protein standard for immunoblotting, immunization and immunoassays.
Reconstitution to filaments: after vimentin is dissolved in 9.5 M urea buffer (see above), protofilaments and filament complexes are obtained by dialyzing the resulting polypeptide solution stepwise to a concentration of 4 M urea and then to low salt condition (50 mM NaCl, 2 mM dithiothreitol, 10 mM Tris-HCl, pH 7.4).
For immunization purposes, the solution can be further dialyzed against PBS (phosphate buffered saline, e.g. Dulbecco's PBS).
- Hatzfeld M. and Franke W.W. (1985). J. Cell Biol. 101, 1826-1841
- Hatzfeld M. et al. (1987). J. Mol. Biol. 197, 237-255
For immunization purposes, the solution can be further dialyzed against PBS (phosphate buffered saline, e.g. Dulbecco's PBS).
- Hatzfeld M. and Franke W.W. (1985). J. Cell Biol. 101, 1826-1841
- Hatzfeld M. et al. (1987). J. Mol. Biol. 197, 237-255
Downloads
Q & A's
There aren't any asked questions yet.
Customer Reviews
Login
FAQs
Our human recombinant vimentin was lyophilized in urea buffer. Vimentin other intermediate filament proteins are insoluble under physiological buffer conditions (like PBS) or low salt conditions. This is a prominent feature of this protein family. There is some solubility in Tris-buffer, however, at very low protein concentration.
To obtain filaments from Cat. No. 62015 we suggest the following procedure:
- Reconsitute the lyophilized material in distilled water to a protein concentration of 1 mg/ml and a urea concentration of 9.5 M.
- Dialyze against 6 M urea buffer (down to 6 M urea vimentin is still present in the monomeric form) for at least 2 h at RT.
- Dialyze against 4M urea buffer (at 4 M urea dimers and protofilaments are formed) for at least 4 h at RT or overnight at 4°C.
- Dialyze to low urea/low salt buffer (at least 2 buffer changes to obtain intermediate filaments) containing 50 mM NaCl, 2 mM dithiothreitol, 10 mM Tris-HCl, pH 7.4, or filament forming buffer (suggested e.g. by Herrmann et al. 1996, J Mol Biol 264, 933-953) containing 25 mM Tris-HCl pH 7.5, 0.1 mM dithiothreitol, 160 mM NaCl- for immunization purposes dialyze to e.g. PBS.
- Dialysis in steps against large buffer volumes is important in order to obtain filament complexes and avoid formation of amorphous aggregates.