Performance Data
AAV-based gene therapy has been growing quickly over the last thirty years. Consequently, there has been an extreme increase in demand from pharma, as well as a positive growth in new technologies in the field. As a result an even larger number of AAV gene therapy products are being transferred from pre-clinical to clinical trials, raising the issue of reliability, reproducibility and comparability for both production and regulatory authorities.
Data Comparability: Each AAV ELISA Kit has to pass a comprehensive quality control. This includes an analysis of intra- and inter-assay variances, as well as lot-to-lot consistencies. This quality control process ensures comparability of results obtained with PROGEN AAV ELISAs.
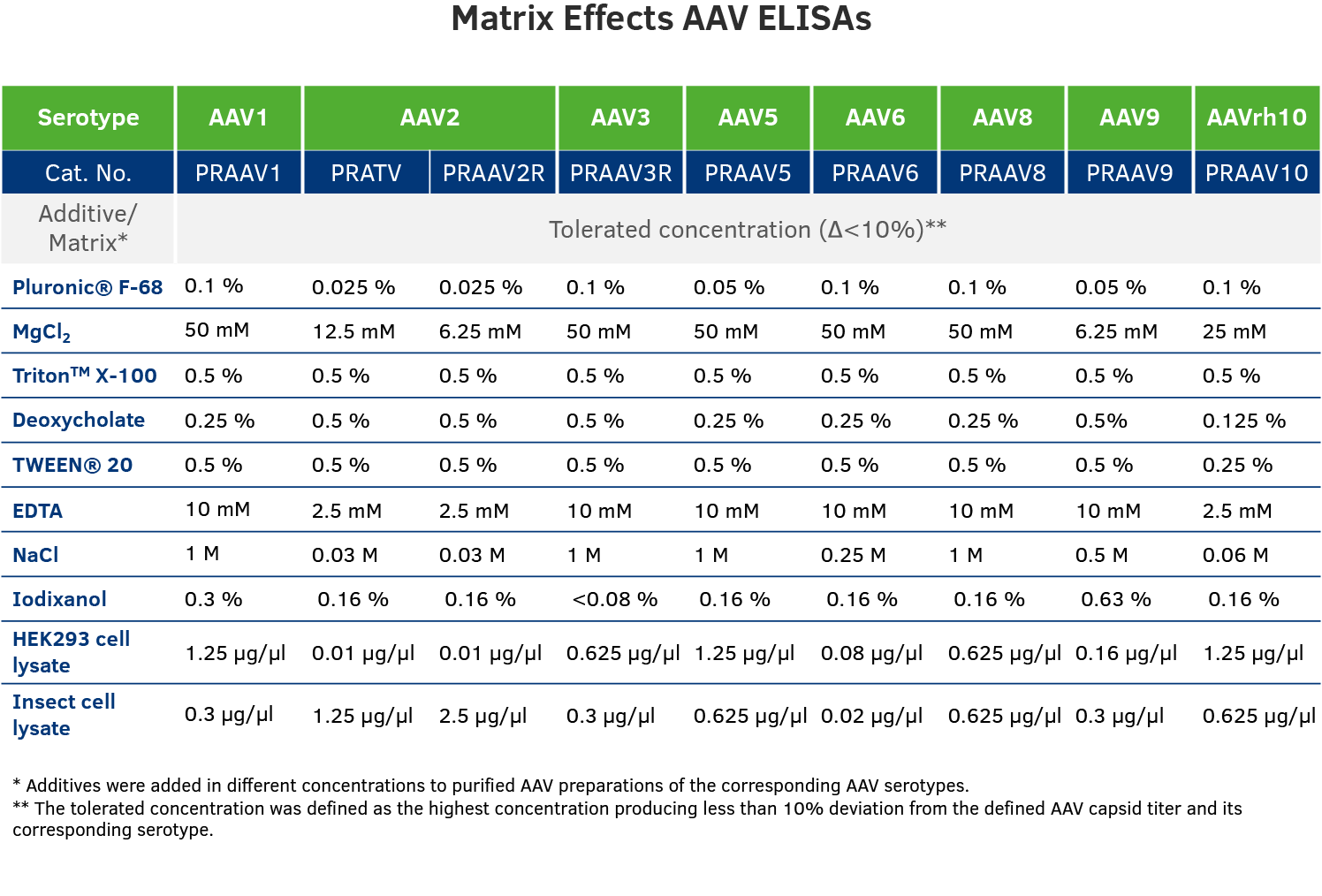
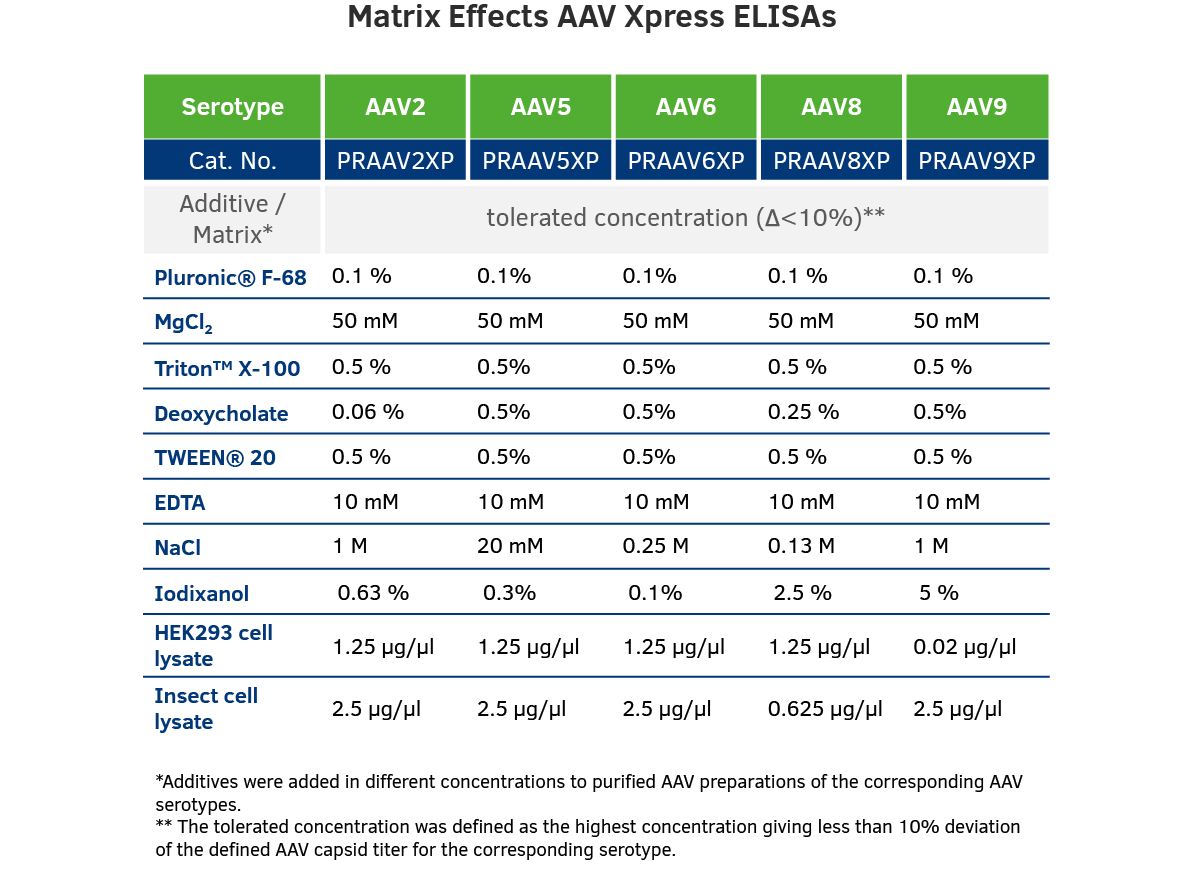
Matrix Effects: There are differences in buffer composition in analyzed samples, due to differences in company production processes. To ensure best possible results, we analyzed tolerated concentrations of different additives in our AAV ELISAs.
Internal Gold Standards: International standard material is only available for serotype AAV2 and AAV8. PROGEN has established internal gold standards to ensure we can provide AAV ELISA kits with consistent quality for each serotype.
Data Comparability
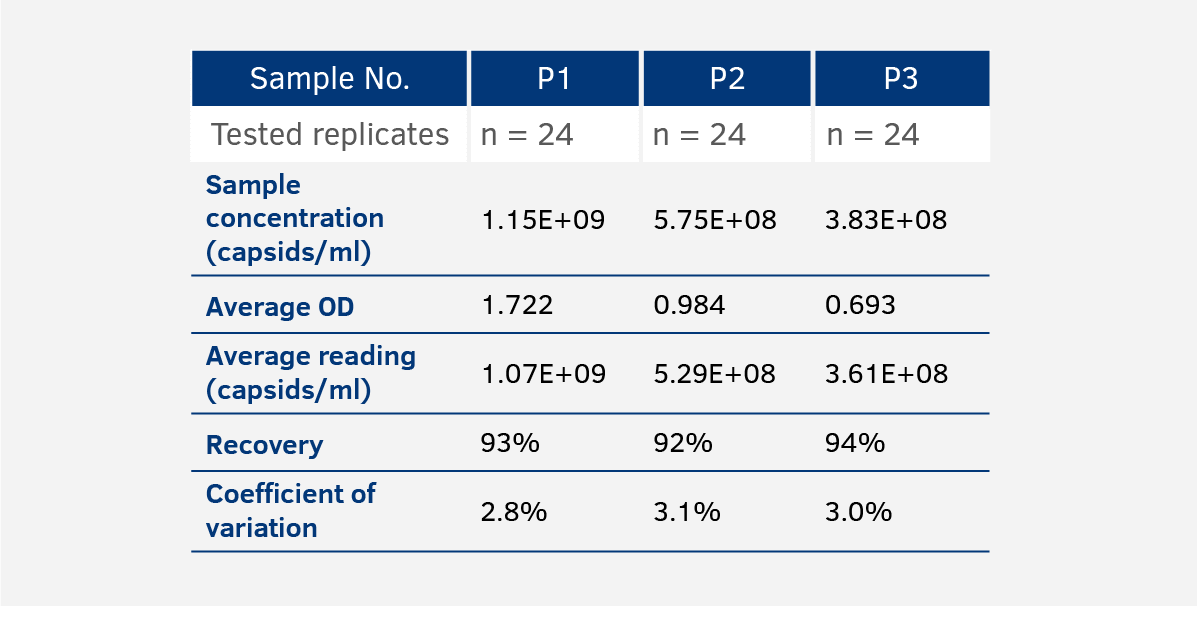
Intra-Assay Variance
The intra-assay variance is tested by applying three samples in 24 replicates to the same microtiter plate in a single run. Recovery has to be within our internal acceptance range of +/- 30% while observed CVs are usually < 5%.
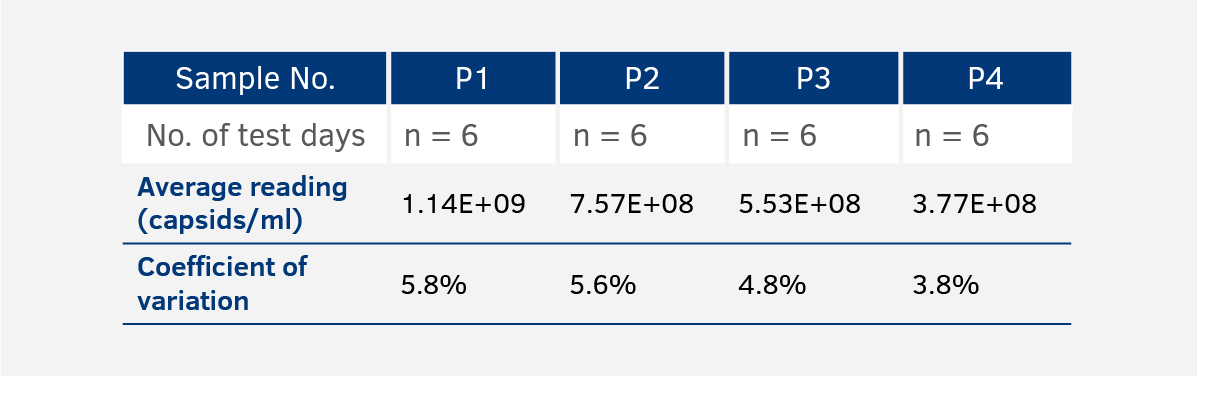
Inter-Assay Variance
The inter-assay variance is determined by measuring a comprehensively characterized internal control in four ELISA plates from the same lot on six different days. Observed CVs are usually < 7%.
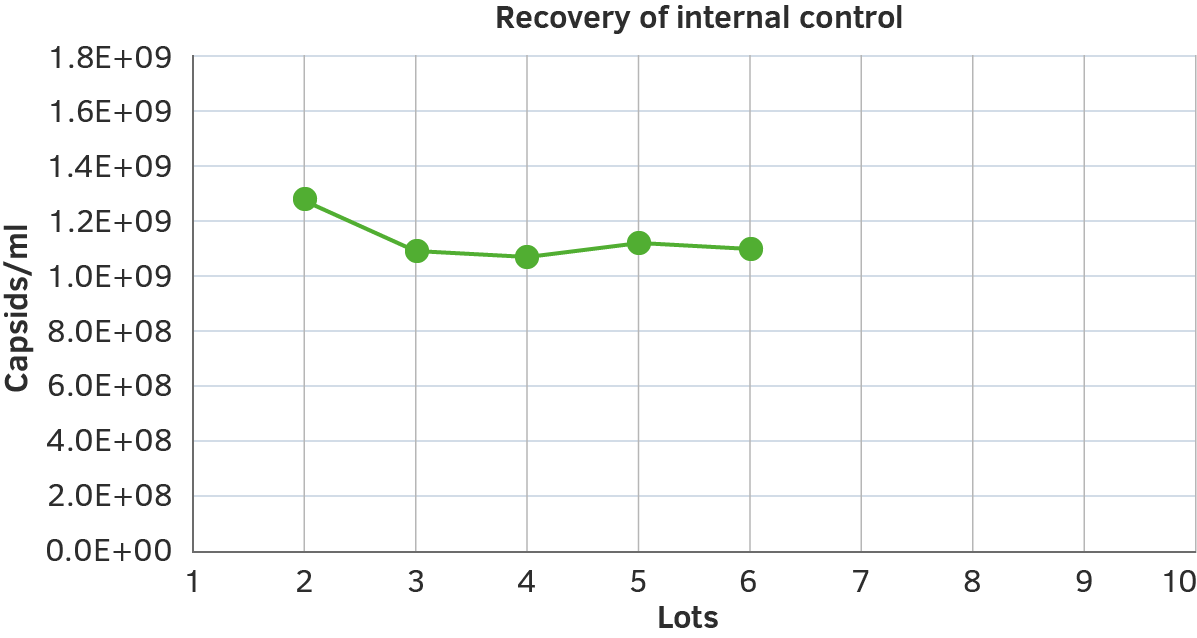
Lot-to-lot Consistency
We focus on ensuring the lot-to-lot consistency of our AAV ELISA kits by using independent internal controls.
NOTE This data is to be used as an example only. Individual serotype and charge specific data is obtained on an individual basis.
Developing an internal gold standard
International standard material is only available for serotype AAV2 and AAV8. To ensure we can provide AAV ELISA kits with consistent quality for each serotype, PROGEN has established internal gold standards. Based on these gold standards each kit is thoroughly calibrated to ensure your data will be consistent, accurate and reproducible.
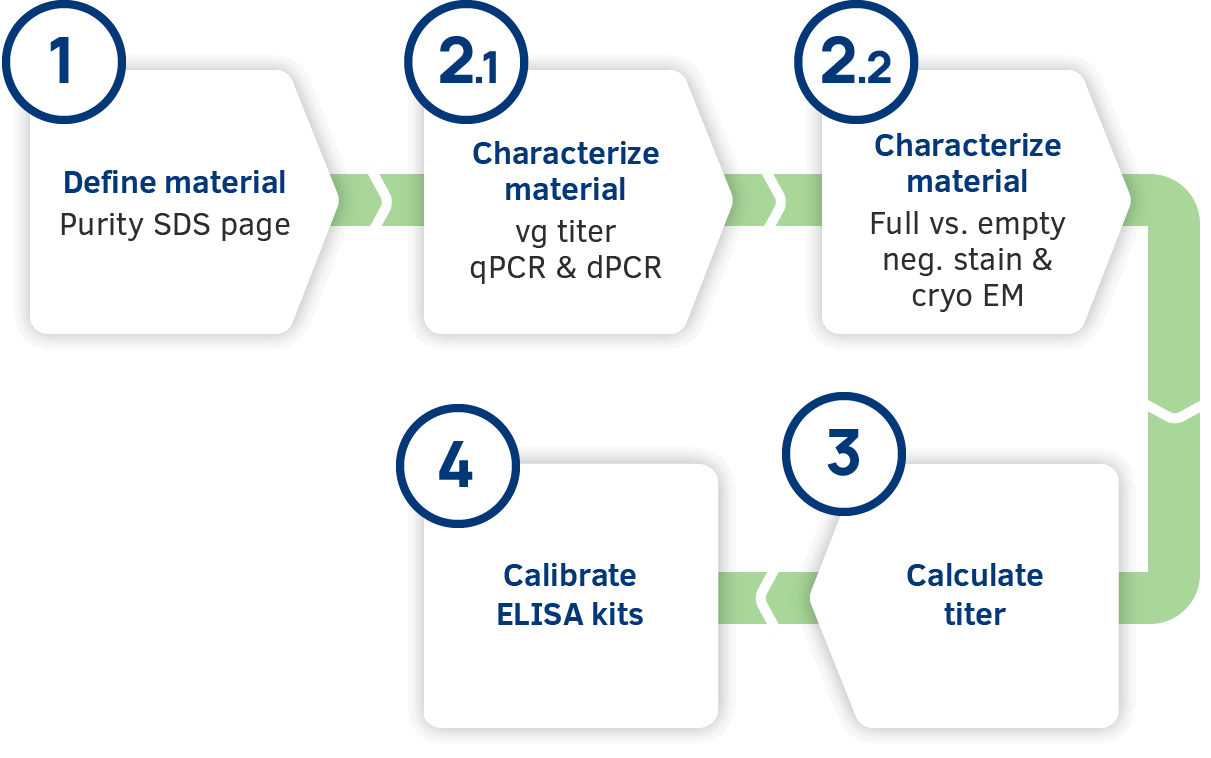
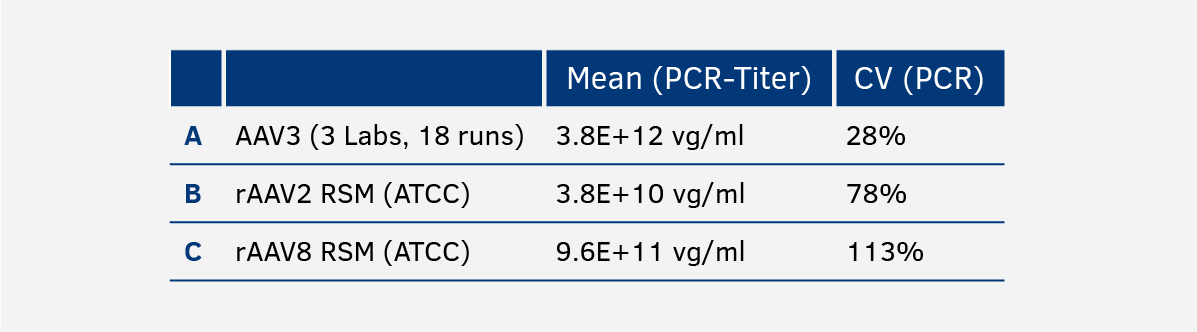
2.1. Genome titer - qPCR/dPCR
Due to the high variabilities of PCR-based methods, the genome titer is determined by three independent laboratories in an average of 15-20 different runs.
2.2. Full vs empty - Neg. stain & cryo EM
Full vs empty AAV capsid ratios are determined by negative stain and cryo EM, which both show similar results for the ratio analysis. Although both methods would be equally suitable for determing the ratio of DNA-filled and empty AAV particles, to ensure the best possible result for the internal gold standard both methods are used.
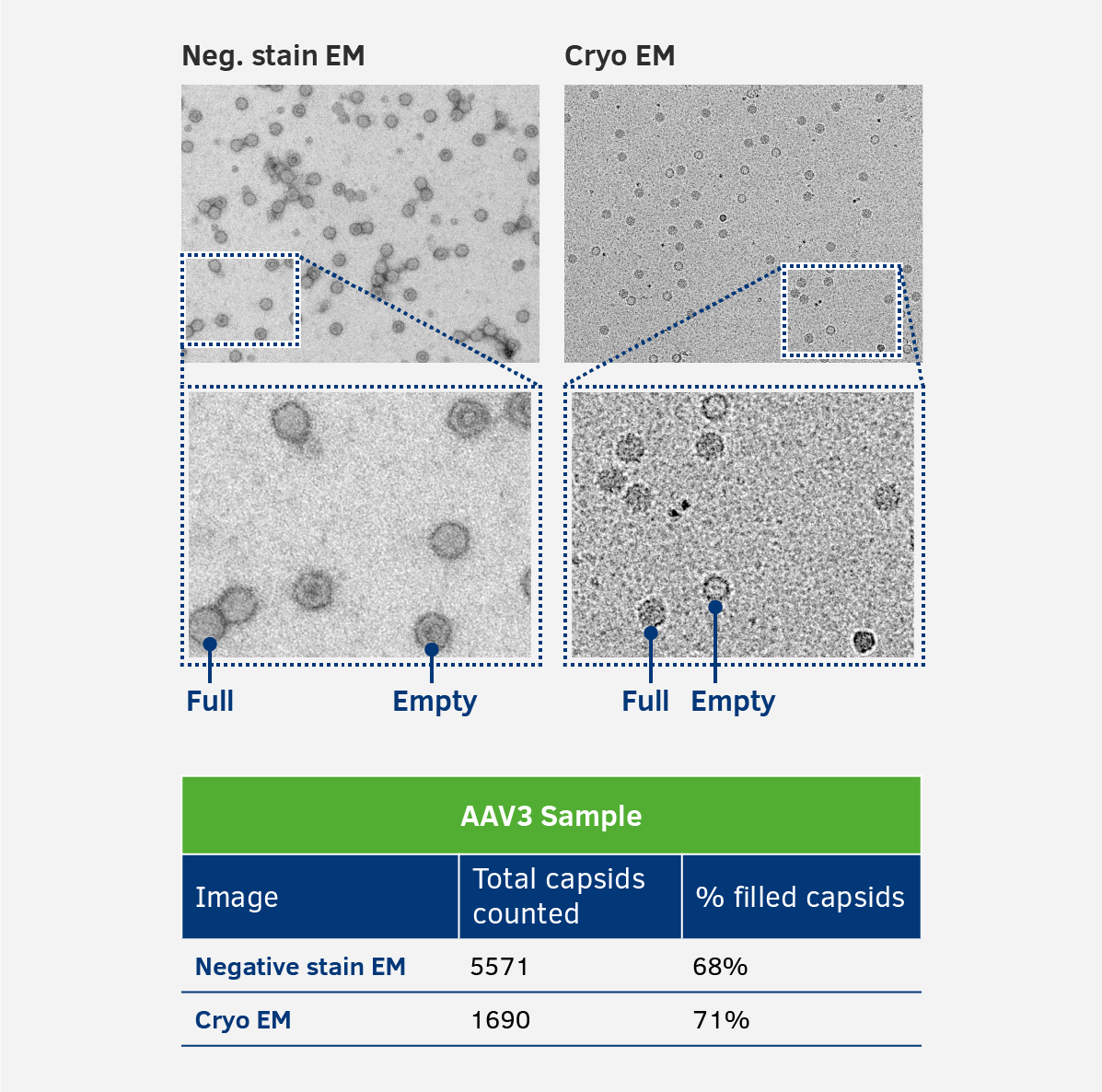
3. Alignment to the internal gold standard
The standards included in the AAV ELISA kits have an assigned, lot-specific titer which has been carefully aligned to the corresponding internal gold standard.
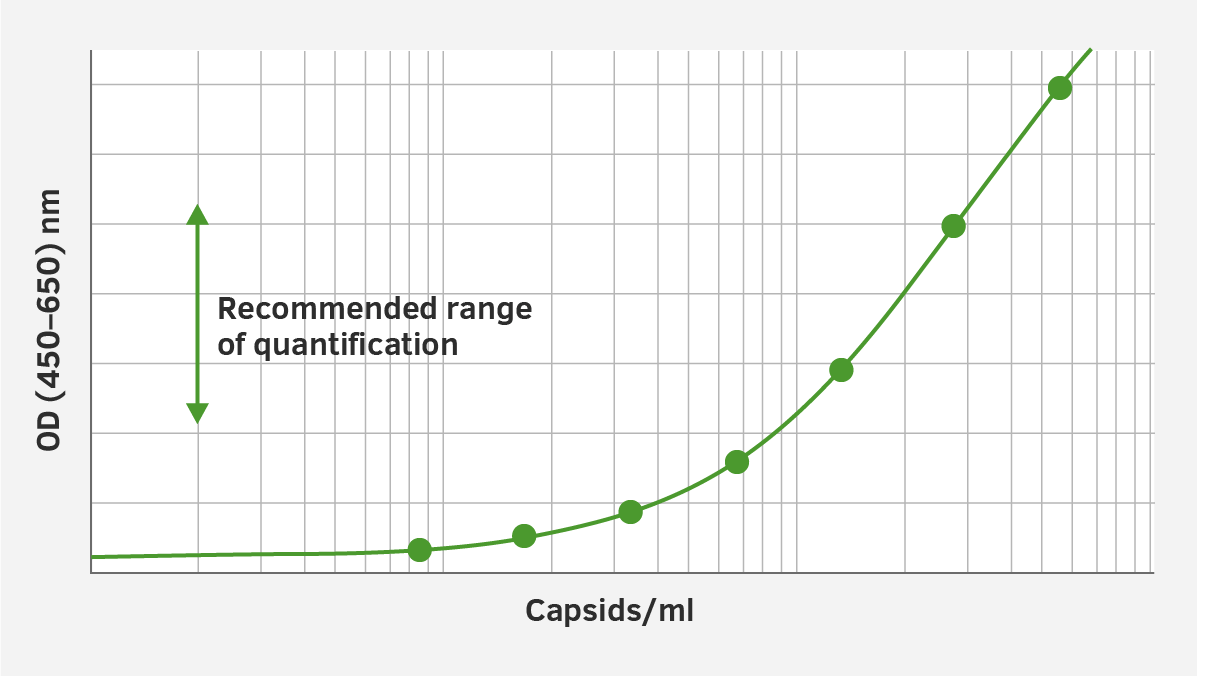
NOTE This data is to be used as an example only. Individual serotype and charge specific data is obtained on an individual basis.



AAV ELISA Controls
Reliable positive controls in detections and quantification assays.